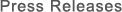Dr. Moriya Tsuji, M.D., Ph.D. is a Professor of Medicine in the Division of Infectious Diseases, Department of Medicine at the Columbia University Irving Medical Center, a world-renowned research institute in the United States. Professor Tsuji and his colleagues have demonstrated for the first time in the world a robust antiviral effect exerted by a Plasmacluster technology developed by Sharp Corporation. The research team has found that the exposure of Plasmacluster ions to an airborne virus of a recent coronavirus variant, SARS-CoV-2 Omicron BA.1 for only 15 minutes resulted in reducing the virus infectious titer*3 by 99.3%.
In this airborne virus study, a highly concentrated solution of the Omicron BA.1 variant, a mutated strain of the novel coronavirus (SARS-CoV-2), was sprayed into a 102L test box*4 in the form of an aerosol followed by releasing Plasmacluster ions (ion density approximately 25,000 pcs/cm3) to verify the effectiveness of reducing airborne virus. The results showed a drastic reduction of the virus infectious titer (99.3% reduction after 15 minutes of exposure), indicating that Plasmacluster technology is vastly effective against mutated and highly infectious airborne Omicron variant.
This study was initiated in April 2020 by an inquiry about Sharp Plasmacluster technology from Columbia University, who was investigating effective infection control technology against the novel coronavirus (SARS-CoV-2). At that time, the number of infected people and deaths had rapidly increased worldwide from the initial onset of the novel coronavirus disease (COVID-19) outbreak in December 2019 and some effective measures were required. Sharp accepted the request made by Columbia University and agreed to fund the study and provided necessary test equipment. Afterwards, the study was conducted independently by the University, leading to the results outlined above.
For more than 20 years since 2000, Sharp has promoted academic marketing*5 to demonstrate the effectiveness of Plasmacluster technology at independent third-party testing institutions in Japan and overseas. Thus far, including this demonstration, 13 testing institutions outside of Japan have proven its effectiveness in suppressing the action of harmful substances such as airborne Serratia bacteria which is a source of hospital-acquired infections (Dr. Melvin W. First, US), airborne influenza viruses (Institute Pasteur, Vietnam), and clinical effectiveness in reducing the risk of tuberculosis infections in tuberculosis hospitals (National Center of Tuberculosis and Lung Diseases, Georgia). Furthermore, the mechanism of action in suppressing viruses, fungi, and bacteria (Professor Gerhard Artmann, Germany) has been clarified. At the same time, Sharp has continued to verify the safety of Plasmacluster technology over the course of many years.
Sharp will continue to actively demonstrate the effectiveness of Plasmacluster technology not only in Japan but also internationally in order to make further social contribution.
Comments from Professor Moriya Tsuji, Columbia University Irving Medical Center
Since 2020, COVID-19 outbreak caused by SARS-CoV-2 virus has spread explosively around the world and become the scourge of humanity. It still continues to spread while continuously mutating to evade our immune system, thus posing an unceasing threat to our society. In addition to vaccination, it is desirable to employ multi-faceted protective measures to prevent wide-spread infection by SARS-CoV-2 virus. In order to conduct a study with the highly infectious Omicron variant, ensuring sufficient safety is indispensable. In this study, a reliable test could be conducted with the maximum size of test box housed in a safety cabinet which does not leak any virus. Consequently, we could conduct the study to prove that Plasmacluster ions robustly reduced airborne SARS-CoV-2 (Omicron variant) virus. It is our strong belief that the application of this Plasmacluster technology as a countermeasure against respiratory virus infections should hold great promise to our society in the future.
*1 In ion-emission air purification technologies against SARS-CoV-2 Omicron BA.1 variant (as of October 13, 2022; based on Sharp findings).
*2 A type of the Omicron strain, a mutated variant of SARS-CoV-2. BA.1 is the main type that was prevalent in Japan from January to March 2022.
*3 Number of infectious virus.
*4 A specially designed test chamber placed inside a safety cabinet.
*5 A marketing method to promote commercialization of products based on verification of scientific data on the effectiveness of a technology in collaboration with leading-edge academic research institutions.
● Plasmacluster and the Plasmacluster logos are registered trademarks of Sharp Corporation.
■ Overview of Airborne Coronavirus Verification Test
• Study conducted by: Prof. Moriya Tsuji (Columbia University Department of Medicine)
Note: Researchers who have contributed in this study: Dr. Yaoxing Huang, Dr. Manoj Nair,
Dr. Kazuya Masuda, Dr. Hiroshi Mohri, Yukiko Tsuji, Patrick Loughlin, Dr. David D Ho
• Test space: A test box having a volume of 102 liters inside a safety cabinet
• Verification test apparatus: Virus testing device equipped with Plasmacluster technology
• Plasmacluster ion density: Approx. 25,000 pcs/cm3 (in the center of test chamber)
• Control study: Comparison using the aforementioned device without ion generation (blowing air only)
• Verification virus: SARS-CoV-2 (Omicron BA.1 variant)
• Test method:
A solution containing SARS-CoV-2 that had been highly concentrated by centrifugation was sprayed into a 102-liters test chamber and exposed to Plasmacluster ions. Subsequently, the airborne virus inside the chamber was recovered. The virus infectious titer was measured using a TCID50 assay*6.
*6 A standard assay involving inoculation of cells with stepwise diluted virus solution and checking the infectivity titer.
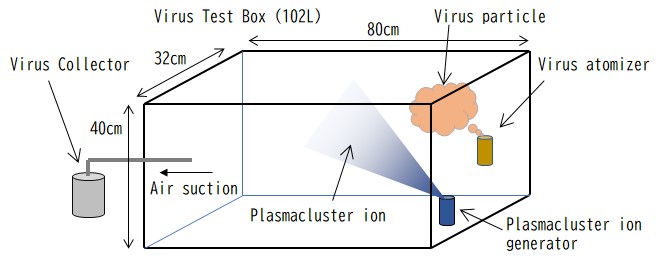
Figure 1. Image of test equipment
• Results:
It was confirmed that the number of infectious virus was reduced by more than 99%
following the exposure to Plasmacluster ions.
|
Virus infectious titer(TCID50/ml) |
Reduction |
|
|
Without Plasmacluster ions |
With Plasmacluster ions |
|
|
8.63x103 |
6.15x101 |
99.3% |
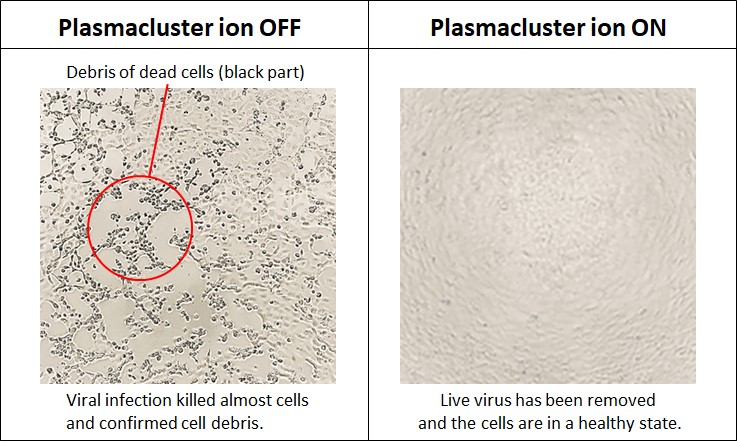
Figure 4. Photomicrograph of cells after incubating for 3 days
with the virus containing solution, which was recovered from the test box
|
Name, Affiliation, Research field |
|
|
|
Moriya Tsuji, MD, PhD Professor of Medicine, Aaron Diamond AIDS Research Center, Division of Infectious Diseases, Department of Medicine, Columbia University Vagelos College of Physicians and Surgeons, Columbia University Irving Medical Center Research field: Infectious disease, Immunology |
Major Plasmacluster technology verification organizations around the world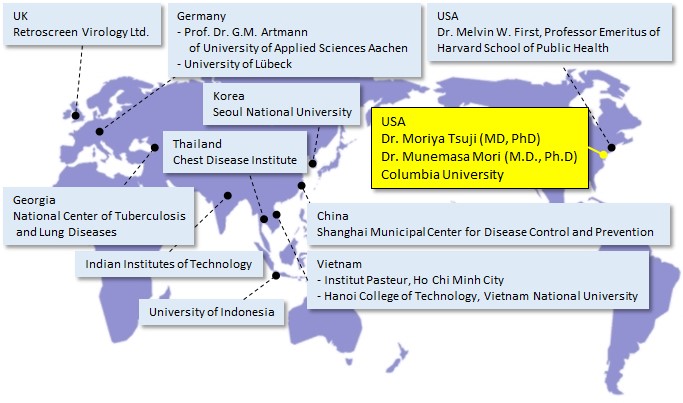
Plasmacluster technology developments to date (overview)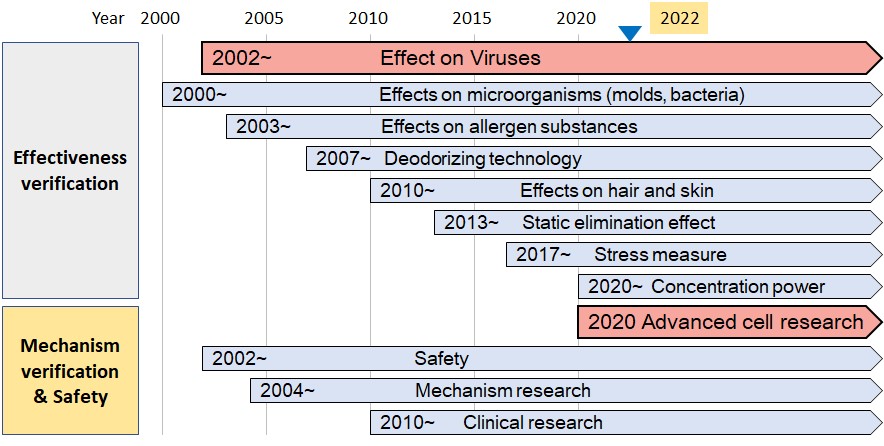
■ Research Institutes That Provided Data for Sharp’s Academic Marketing
|
Target |
Testing and Verification Organization |
|
Viruses |
Columbia University Irving Medical Center, USA |
|
Kitasato Research Center of Environmental Sciences | |
|
Seoul National University | |
|
Shanghai Municipal Center for Disease Control and Prevention, China | |
|
Kitasato Institute Medical Center Hospital | |
|
Retroscreen Virology, Ltd., UK | |
|
Shokukanken Inc. | |
|
University of Indonesia | |
|
Hanoi College of Technology, Vietnam National University, Vietnam | |
|
Institut Pasteur, Ho Chi Minh City, Vietnam | |
|
National Research Center for the Control and Prevention of Infectious Diseases, Institute of Tropical Medicine, Nagasaki University | |
|
Department of Microbiology, Shimane University, Faculty of Medicine | |
|
Evaluation of effects on cells |
Columbia University Irving Medical Center, USA |
|
Efficacy proven in clinical trials |
Graduate School of Medicine, University of Tokyo / Public Health Research Foundation |
|
Faculty of Science and Engineering, Chuo University / Clinical Research Support Center, University Hospital, University of Tokyo | |
|
Animal Clinical Research Foundation | |
|
Soiken Inc. | |
|
School of Bioscience and Biotechnology, Tokyo University of Technology | |
|
National Trust Co., Ltd. / HARG Treatment Center | |
|
National Center of Tuberculosis and Lung Diseases, Georgia | |
|
Dentsu ScienceJam Inc. | |
|
Littlesoftware Inc. | |
|
National Institute of Fitness and Sports in Kanoya | |
|
Kyushu Sangyo University, Department of Sport Science and Health, Faculty of Human Sciences | |
|
Shibaura Institute of Technology, College of Systems Engineering and Science, Department of Machinery and Control Systems | |
|
Fungi |
Ishikawa Health Service Association |
|
University of Lübeck, Germany | |
|
Professor Gerhard Artmann, Aachen University of Applied Sciences, Germany | |
|
Japan Food Research Laboratories | |
|
Shokukanken Inc. | |
|
Shanghai Municipal Center for Disease Control and Prevention, China | |
|
Biostir Inc. | |
|
Medical Mycology Research Center, Chiba University | |
|
Bacteria |
Ishikawa Health Service Association |
|
Shanghai Municipal Center for Disease Control and Prevention, China | |
|
Kitasato Research Center of Environmental Sciences | |
|
Kitasato Institute Medical Center Hospital | |
|
Dr. Melvin W. First, Professor Emeritus, Harvard School of Public Health, US | |
|
Animal Clinical Research Foundation | |
|
University of Lübeck, Germany | |
|
Professor Gerhard Artmann, Aachen University of Applied Sciences, Germany | |
|
Japan Food Research Laboratories | |
|
Shokukanken Inc. | |
|
Chest Disease Institute, Thailand | |
|
Biostir Inc. | |
|
Allergens |
Graduate School of Advanced Sciences of Matter, Hiroshima University |
|
Department of Biochemistry and Molecular Pathology, Graduate School of Medicine, Osaka City University | |
|
Safety |
LSI Medience Corporation |
|
Odors, pet smells |
Boken Quality Evaluation Institute |
|
Animal Clinical Research Foundation | |
|
Skin beautifying effects |
School of Bioscience and Biotechnology, Tokyo University of Technology |
|
Hair beautifying effects |
Saticine Medical Co., Ltd. |
|
C.T.C Japan Ltd. | |
|
Plant |
Facility of Agriculture, Shizuoka University |
|
Hazardous chemical substances |
Sumika Chemical Analysis Service Ltd. |
|
Indian Institutes of Technology Delhi | |
|
Working mechanism of inhibitory effects on viruses, fungi, and bacteria |
Professor Gerhard Artmann, Aachen University of Applied Sciences, Germany |
|
Working mechanism of inhibitory effects on allergens |
Graduate School of Advanced Sciences of Matter, Hiroshima University |
|
Working mechanism of skin moisturizing (water molecule coating) effect |
Research Institute of Electrical Communication, Tohoku University |


 Choose
Choose