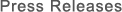Dr. Munemasa Mori, Principal Investigator and Assistant Professor of Medicine Columbia Center for Human Development, Department of Medicine at Columbia University in New York, U.S., is an expert in respiratory medicine and stem cell research. Dr. Mori and his investigative team have verified the effect of Sharp Corporation’s Plasmacluster technology on long-term cultured human airway stem cells and confirmed its potential to decrease viscous airway mucus secretion, a problem in asthma patients, suggesting that this technology may help alleviate respiratory tract symptoms related to airway conditions such as asthma.
Airway epithelial cells*2 line the luminal surface of the respiratory tract from the nasal cavity to the lungs. The airway epithelium plays a critical role in mucus clearance by expelling foreign substances through the mucus secretion from secretory cells and the unidirectional beating of motile ciliated cells. The study design investigated the effects of ions on the respiratory tract. Briefly, human airway tissue stem cells were first induced to differentiate into ciliated and secretory epithelial cells over the course of one month. The cultured cells formed a sheet-like structure and were then exposed to Plasmacluster ions for a maximum of 24 hours.
Dr. Mori’s team did not observe obvious gross morphological changes in the cells or a sign of cell damage. Instead, quantitative gene expression analysis*3 suggested a decrease in the marker linked with highly-viscous mucus associated with impaired breathing in the respiratory tract of asthma patients and an increase in the marker linked with the smooth secretory protein related to low-viscosity mucus that helps improve breathing. These changes may lead to symptom relief by improving airway mucus secretion, a significant problem in asthma patients. To date, Sharp has demonstrated the effectiveness of Plasmacluster technology in suppressing mite allergens that cause airway problems. Since this test was performed with equipment where no allergens such as dust mites and pollen were present, it suggests that Plasmacluster ions' effects may also contribute to benefit cells directly.
Sharp will further continue to verify the effects on cells and their mechanisms.
In addition to measures to counter SARS-CoV-2, Dr. Mori was interested in the overall effects of Plasmacluster ions on the respiratory system. Dr. Mori's team conducted the experiments independently at Columbia University using Plasmacluster technology equipment provided by Sharp.
For more than 20 years, Sharp has promoted academic marketing*4 to demonstrate the effectiveness of Plasmacluster technology at independent third-party testing institutions in Japan and overseas. Thus far, numerous independent testing organizations have proven its efficacy in suppressing the activity of harmful substances, including mite allergens, as well as clinical effectiveness in reducing bronchial inflammation levels in children with asthma and prevention and treatment of atopic dermatitis in mice. At the same time, Sharp has continued to verify the safety of Plasmacluster technology over the course of many years.
With the discovery of potential new effects of Plasmacluster technology, which may lead to relieving asthma symptoms in the human respiratory tract, Sharp will continue to demonstrate the effectiveness of Plasmacluster technology actively.
Comments from Dr. Munemasa Mori, Assistant Professor, Columbia University Department of Medicine
I was interested in how Plasmacluster technology, with its great potential to reduce viruses, could affect the human respiratory system. So my investigative teams and I designed and conducted the experiment. We exposed Plasmacluster ions directly to airway epithelial cells differentiated from human tissue-specific stem cells and observed the changes in the mucus markers. This is significant insight because it may lead to a relief in asthma symptoms at the cellular level. We are excited to investigate the further evaluation of the effects of Plasmacluster ions on airway epithelial cells using patient’s derived cells, the long-term effects, exploring the potential mechanism, and so on.
I look forward to the technological advancements of Plasmacluster technology that can ultimately lead to their contribution to new therapies.
*1 The research results of ion emission type air purification technology suggest the possibility of alleviating asthma symptoms by acting on human airway epithelial cells to alter the mucus markers involved in mucus production. (as of October 13, 2022, based on Sharp findings).
*2 Cells are lining the respiratory tract. They function as an outer layer of the body and are composed of multiple cell types, such as ciliated and mucous cells.
*3 A method of investigating the internal state of cells, an analysis method that examines the functions and reactions of genes.
*4 A marketing method to promote the commercialization of products based on verification of scientific data on the effectiveness of technology in collaboration with leading-edge academic research institutions.
●Plasmacluster and the Plasmacluster logos are registered trademarks of Sharp Corporation.
■ Overview of Test to Investigate the Effects of Plasmacluster Ions on Human Cells
• Test conducted by: Dr. Munemasa Mori (Columbia University Department of Medicine)
Note: Researchers who cooperated in this test: Dr. Youngmin Hwang, Dr. Anri Sawada,
Dr. Yuko Shimamura, Dr. Tatsuya Nagano, Dr. Akihiro Miura, Dr. Junichi Tanaka, and Dr. Danting Cao
• Test space: Inside a 12-liter test chamber installed within the cell culture incubator
• Verification test apparatus: Testing device equipped with Plasmacluster technology
• Plasmacluster ion density: At sample position inside air duct: approx. 100,000 pcs/cm3
• Control test: Comparison using the abovementioned device without ion generation
• Verification cells: Respiratory tract epithelial cells that have been induced to differentiate from human tissue stem cells for approximately one month and then cultured into a sheet
• Test method:
1) Expose human respiratory tract epithelial cells placed in a test chamber to Plasmacluster ions for a certain period
2) Observe the status of cell growth and morphology after exposure and perform quantitative gene expression analysis by qPCR
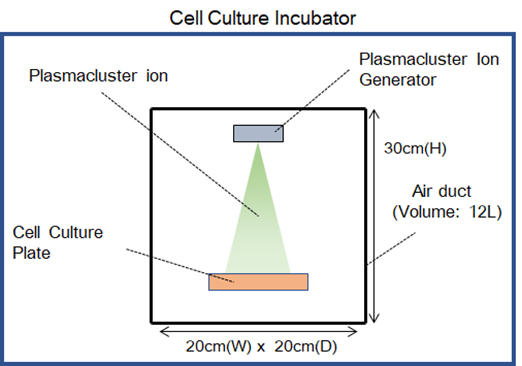
Figure 1: Test equipment image
• Results:
1.Observation results of cells (24-hour exposure to Plasmacluster ions)
Noticeable gross-morphological changes or cell damages of human respiratory tract epithelial cells were not confirmed after exposure to Plasmacluster ions for 24 hours.
• Observation of cell surface:
There was no change on the cell's surface exposed to Plasmacluster ions, although abnormalities indicating damage were shown on the surface exposed to hydrogen peroxide solution.
• In the ROS signaling analysis using fluorescent dyes:
There was no cell damage caused by oxidative reaction exposed to Plasmacluster ions, although apparent ROS signaling activation and cell damages were observed exposed to hydrogen peroxide solution
Table 1. Microscopy image of cell surface
2.Analysis results of human respiratory tract epithelial cells (gene expression test)
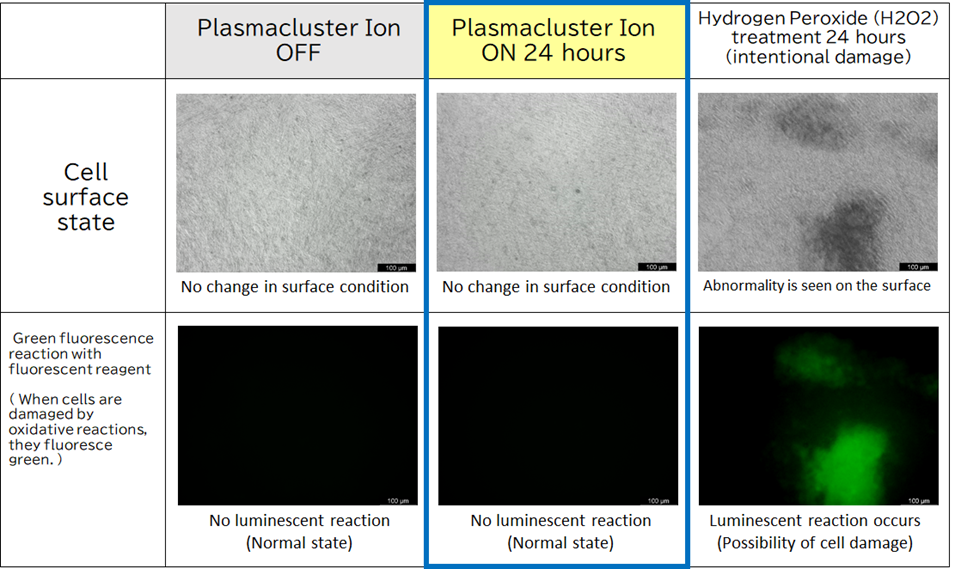
The changes in the following protein markers in airway epithelial cells exposed to Plasmaculuster ions were confirmed. These changes may improve mucus secretion in the airways, a severe problem in asthma patients, and may lead to symptom relief.
• A decrease in MUC5AC was confirmed after exposure to Plasmacluster ions.
MUC5AC is a protein marker associated with impaired breathing by increasing high-viscous mucus in the respiratory tract of asthma patients.
• An increase in SCGB1A1 was confirmed after exposure to Plasmacluster ions.
SCGB1A1 is a protein marker associated with helping to improve breathing by increasing low-viscosity mucus.
Figure 2: Graph showing the effect of Plasmacluster ions on proteins involved in mucus increase/decrease.
|
Name, Affiliation, Research field |
|
|
|
Munemasa Mori, M.D., Ph.D. Assistant Professor of Medicine, Columbia Center for Human Development, Division of Pulmonary, Allergy, and Critical Care Medicine, Department of Medicine, Columbia University Vagelos College of Physicians and Surgeons, Columbia University Irving Medical Center Research field: Pulmonology, Stem cell biology |
Major Plasmacluster technology verification organizations around the world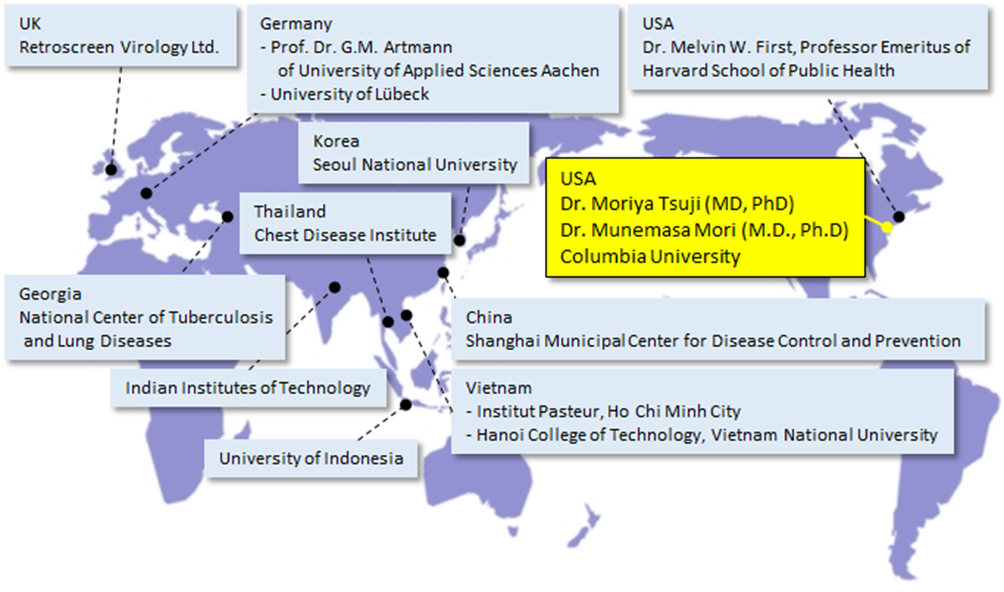
Plasmacluster technology developments to date (overview)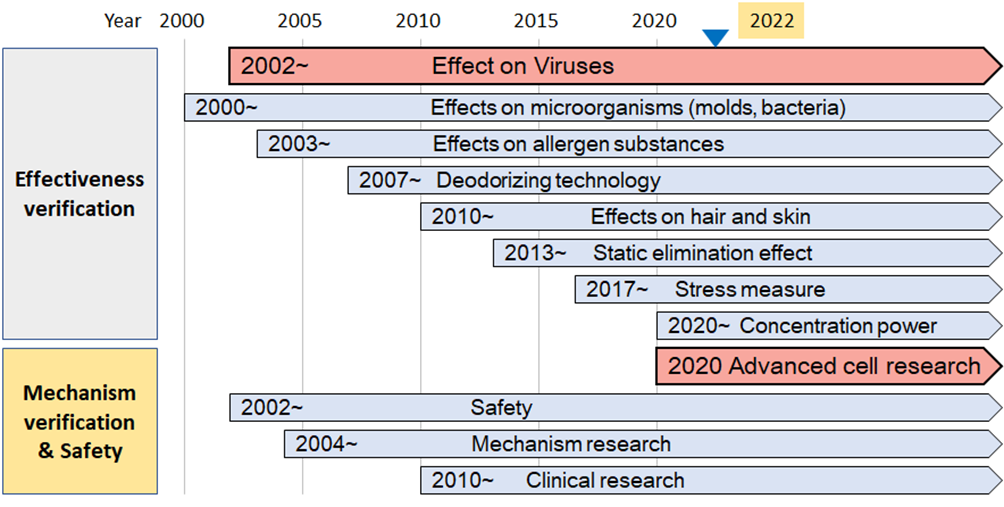
■ Research Institutes That Provided Data for Sharp’s Academic Marketing
|
Target |
Testing and Verification Organization |
|
Viruses |
Columbia University Irving Medical Center, USA |
|
Kitasato Research Center of Environmental Sciences | |
|
Seoul National University | |
|
Shanghai Municipal Center for Disease Control and Prevention, China | |
|
Kitasato Institute Medical Center Hospital | |
|
Retroscreen Virology, Ltd., UK | |
|
Shokukanken Inc. | |
|
University of Indonesia | |
|
Hanoi College of Technology, Vietnam National University, Vietnam | |
|
Institut Pasteur, Ho Chi Minh City, Vietnam | |
|
National Research Center for the Control and Prevention of Infectious Diseases, Institute of Tropical Medicine, Nagasaki University | |
|
Department of Microbiology, Shimane University, Faculty of Medicine | |
|
Evaluation of effects on cells |
Columbia University Irving Medical Center, USA |
|
Efficacy proven in clinical trials |
Graduate School of Medicine, University of Tokyo / Public Health Research Foundation |
|
Faculty of Science and Engineering, Chuo University / Clinical Research Support Center, University Hospital, University of Tokyo | |
|
Animal Clinical Research Foundation | |
|
Soiken Inc. | |
|
School of Bioscience and Biotechnology, Tokyo University of Technology | |
|
National Trust Co., Ltd. / HARG Treatment Center | |
|
National Center of Tuberculosis and Lung Diseases, Georgia | |
|
Dentsu ScienceJam Inc. | |
|
Littlesoftware Inc. | |
|
National Institute of Fitness and Sports in Kanoya | |
|
Kyushu Sangyo University, Department of Sport Science and Health, Faculty of Human Sciences | |
|
Shibaura Institute of Technology, College of Systems Engineering and Science, Department of Machinery and Control Systems | |
|
Fungi |
Ishikawa Health Service Association |
|
University of Lübeck, Germany | |
|
Professor Gerhard Artmann, Aachen University of Applied Sciences, Germany | |
|
Japan Food Research Laboratories | |
|
Shokukanken Inc. | |
|
Shanghai Municipal Center for Disease Control and Prevention, China | |
|
Biostir Inc. | |
|
Medical Mycology Research Center, Chiba University | |
|
Bacteria |
Ishikawa Health Service Association |
|
Shanghai Municipal Center for Disease Control and Prevention, China | |
|
Kitasato Research Center of Environmental Sciences | |
|
Kitasato Institute Medical Center Hospital | |
|
Dr. Melvin W. First, Professor Emeritus, Harvard School of Public Health, US | |
|
Animal Clinical Research Foundation | |
|
University of Lübeck, Germany | |
|
Professor Gerhard Artmann, Aachen University of Applied Sciences, Germany | |
|
Japan Food Research Laboratories | |
|
Shokukanken Inc. | |
|
Chest Disease Institute, Thailand | |
|
Biostir Inc. | |
|
Allergens |
Graduate School of Advanced Sciences of Matter, Hiroshima University |
|
Department of Biochemistry and Molecular Pathology, Graduate School of Medicine, Osaka City University | |
|
Safety |
LSI Medience Corporation |
|
Odors, pet smells |
Boken Quality Evaluation Institute |
|
Animal Clinical Research Foundation | |
|
Skin beautifying effects |
School of Bioscience and Biotechnology, Tokyo University of Technology |
|
Hair beautifying effects |
Saticine Medical Co., Ltd. |
|
C.T.C Japan Ltd. | |
|
Plant |
Facility of Agriculture, Shizuoka University |
|
Hazardous chemical substances |
Sumika Chemical Analysis Service Ltd. |
|
Indian Institutes of Technology Delhi | |
|
Working mechanism of inhibitory effects on viruses, fungi, and bacteria |
Professor Gerhard Artmann, Aachen University of Applied Sciences, Germany |
|
Working mechanism of inhibitory effects on allergens |
Graduate School of Advanced Sciences of Matter, Hiroshima University |
|
Working mechanism of skin moisturizing (water molecule coating) effect |
Research Institute of Electrical Communication, Tohoku University |


 Choose
Choose